Your Location:Home >Products >Functional intermediates >30069-65-9

Product Details
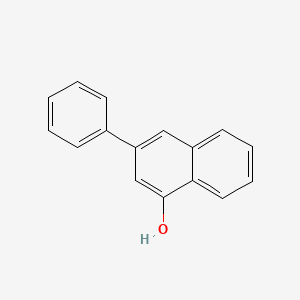
3-Phenylnaphthalen-1-ol is an organic compound consisting of a naphthalene core with a phenyl substituent at the 3-position and a hydroxyl group at the 1-position. This structural arrangement confers interesting photophysical and biological properties to the molecule. The phenyl group introduces additional conjugation and influences the electronic characteristics of the naphthalene system, while the hydroxyl group provides opportunities for further chemical modification and functionalization. 3-Phenylnaphthalen-1-ol has found applications in the development of luminescent materials, organic light-emitting diodes (OLEDs), and as a scaffold in medicinal chemistry for the design of biologically active compounds. The combination of the naphthalene and phenyl moieties, along with the hydroxyl group, makes 3-Phenylnaphthalen-1-ol a valuable building block in organic synthesis and materials science.
Next, the preformed 3-phenylnaphthalen-1-ol 6 was subjected to reaction with 2-phenylindole … This indicates that 3-phenylnaphthalen-1-ol is not an intermediate for the generation of 3, …
This research successfully achieved a Cu(II)-catalyzed 6π-photocyclization of non-6πsubstrates. The photoenolization converts ortho-alkylphenyl alkynl ketones into a triene-type intermediate which undergoes the subsequent 6π-photocyclization to give napht
… into the formation of 4, the preformed 3-phenylnaphthalen-1-ol 6 was treated with indole 4 d … It did not give the desired product 4 l (Scheme 5a), indicating that 3-phenylnaphthalen-1-ol …
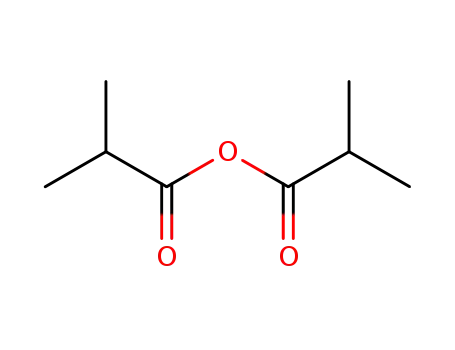
2-Methylpropionic anhydride

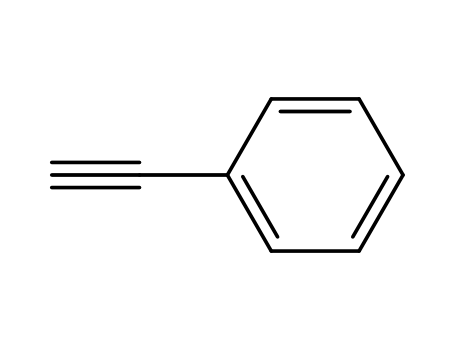
phenylacetylene

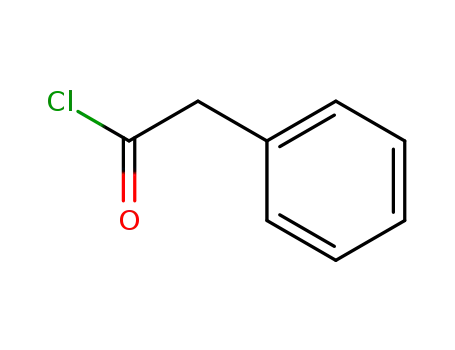
phenylacetyl chloride

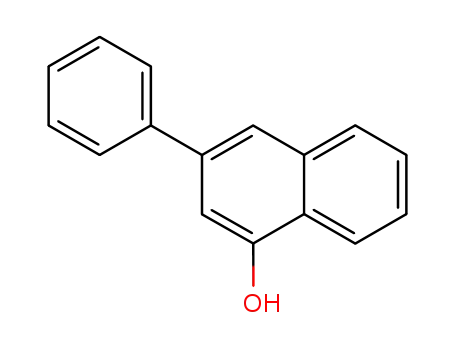
3-phenylnaphthalen-1-ol

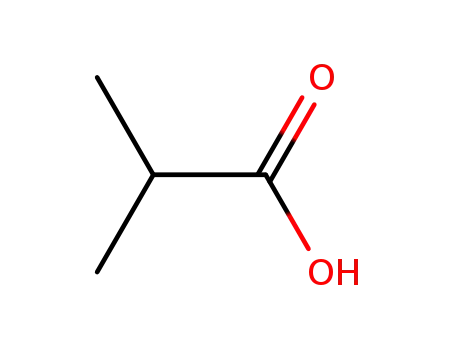
isobutyric Acid
| Conditions | Yield |
|---|---|
|
2-Methylpropionic anhydride; phenylacetylene; phenylacetyl chloride; at 190 ℃; for 48h; Inert atmosphere;
With potassium hydroxide; In water; at 100 ℃; for 13 - 15h;
|
70% |
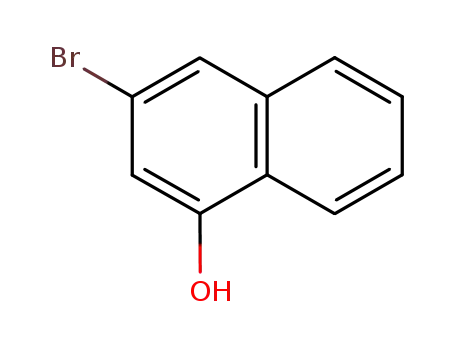
3-bromo-1-hydroxynaphthalene

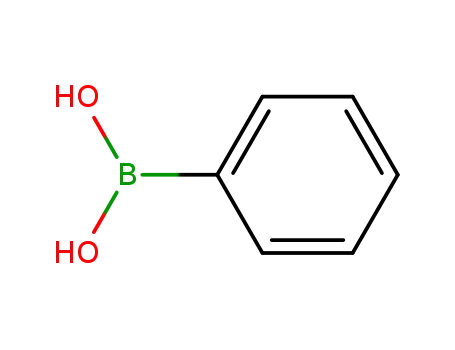
phenylboronic acid


3-phenylnaphthalen-1-ol
| Conditions | Yield |
|---|---|
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; toluene; for 16h; Reflux;
|
75% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; toluene; for 16h; Reflux;
|
75% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; toluene; for 16h; Reflux;
|
75% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; toluene; for 16h; Reflux;
|
75% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; at 65 ℃; for 18h;
|
73% |
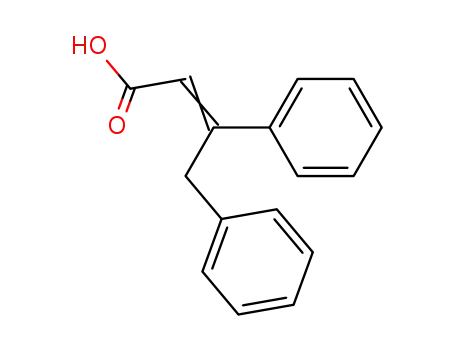
3,4-diphenyl-crotonic acid
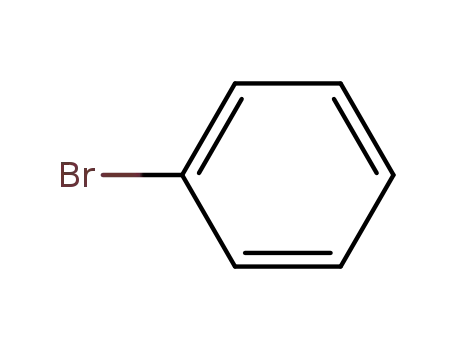
bromobenzene
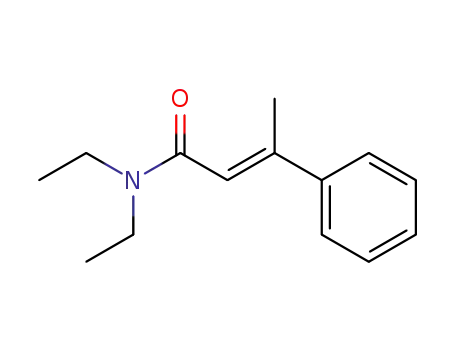
(E)-N,N-diethyl-3-phenylbut-2-enamide
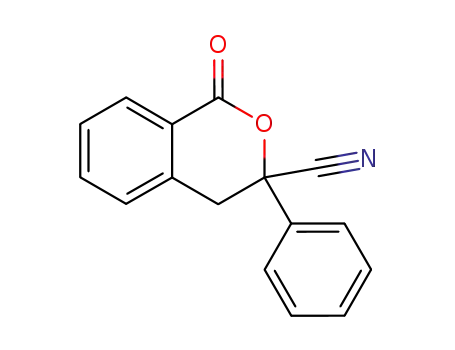
3,4-dihydro-3-cyano-3-phenyl-1H-2-benzopyran-1-one
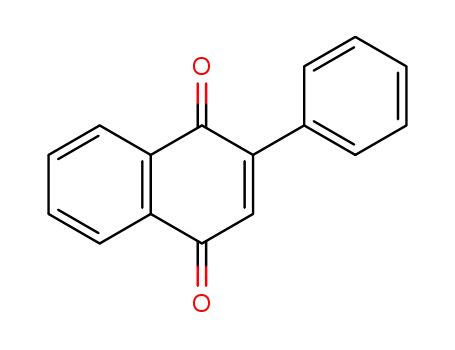
2-phenyl[1,4]naphthoquinone
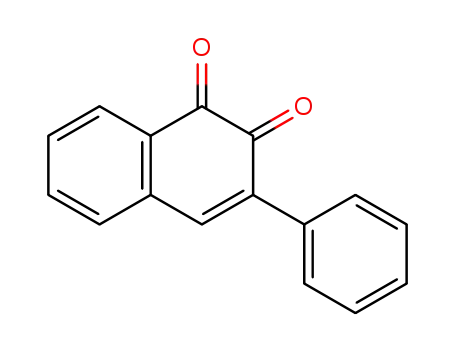
3-phenylnaphthalene-1,2-dione
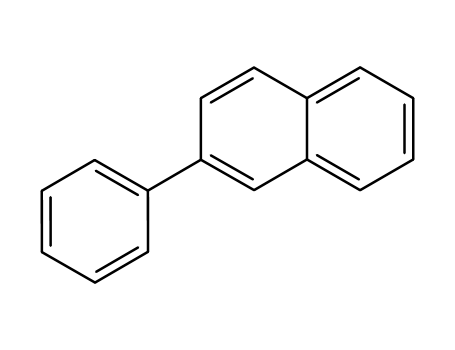
2-phenylnaphthalene
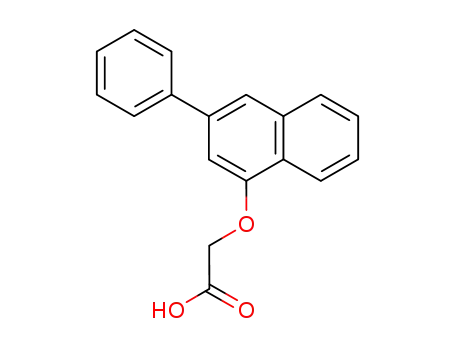
<3-Phenyl-naphthyl-(1)-oxy>-essigsaeure
CAS:120-74-1
Molecular Formula:C<sub>8</sub>H<sub>10</sub>O<sub>2</sub>
Molecular Weight:138.16
CAS:13029-09-9
Molecular Formula:C<sub>12</sub>H<sub>8</sub>Br<sub>2</sub>
Molecular Weight:312