Your Location:Home >Products >Organic Chemistry >1462-96-0

Product Details
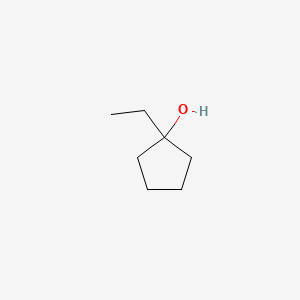
1-Ethylcyclopentanol (1-ECP) is a cyclic alcohol that is widely used in the synthesis of organic compounds.
Isomeric SMILES: CCC1(CCCC1)O
InChIKey: LPCWIFPJLFCXRS-UHFFFAOYSA-N
InChI: InChI=1S/C7H14O/c1-2-7(8)5-3-4-6-7/h8H,2-6H2,1H3
Oxidation of 1-Ethylcyclopentanol with LTA. A mixture of 0.01 mole of 1-ethylcyclopentanol, … We obtained 9 mmoles of 1-ethylcyclopentanol and 0.5 mmole of ethyl butyl ketone. Infrared …
Methods of synthesizing compounds using CO2 as a directing group for C—H functionalization, and compounds made thereby, are described.
The invention relates to the field of optical materials and in particular to a diester acid protection structure monomer and a preparation method thereof. Due to a diester long side chain, the photoresist has a better film-forming property; due to the fact that small-size and high-acid-sensitivity groups hung outside can improve the deprotection reaction efficiency in the photoetching process, the quality of a photoetched product is improved; and in addition, the diester acid protection structure monomer prepared through the process method has high yield and purity, and the performance of the photoresist is further guaranteed.
The expanding “toolbox” of biocatalysts opens new opportunities to redesign synthetic strategies to target molecules by incorporating a key enzymatic step into the synthesis. Significantly, we show that the reversible enzymatic process can power the shuttling of amine functionality across a molecular framework, providing access to the desired aza-Michael products.
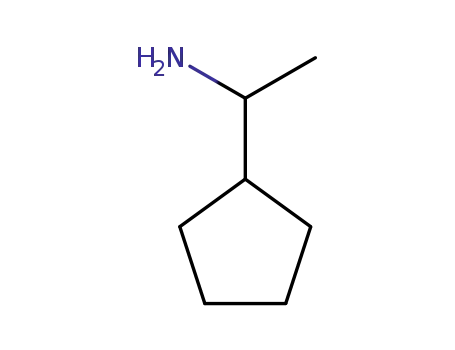
1-cyclopentylethan-1-amine

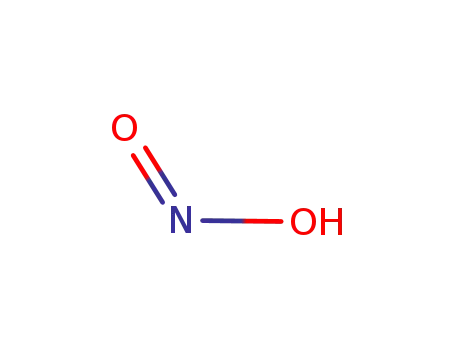
cis-nitrous acid

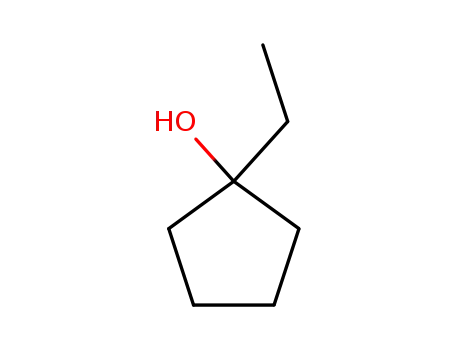
1-ethylcyclopentanol

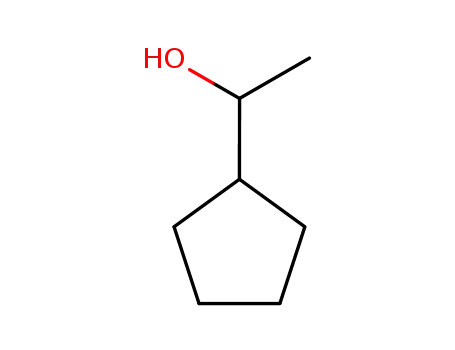
1-cyclopentylethan-1-ol

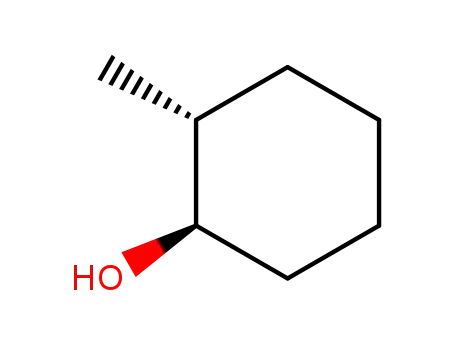
(+/-)-trans-2-methylcyclohexanol
| Conditions | Yield |
|---|---|
|
|
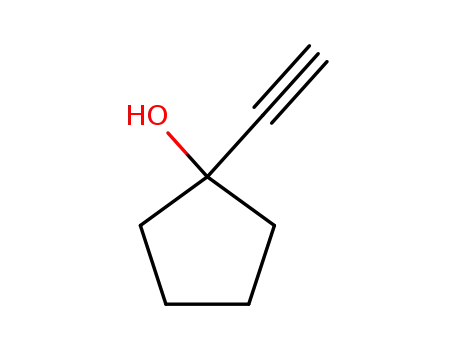
1-ethynylcyclopentanol


1-ethylcyclopentanol
| Conditions | Yield |
|---|---|
|
With magnesium; palladium on activated charcoal; In methanol; Ambient temperature;
|
90% |
|
With 5%-palladium/activated carbon; hydrogen; In methanol; at 80 ℃; under 22502.3 Torr; Autoclave;
|
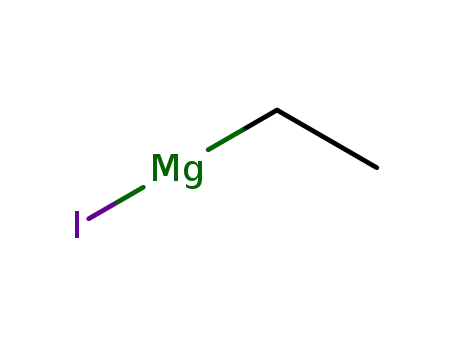
ethylmagnesium iodide
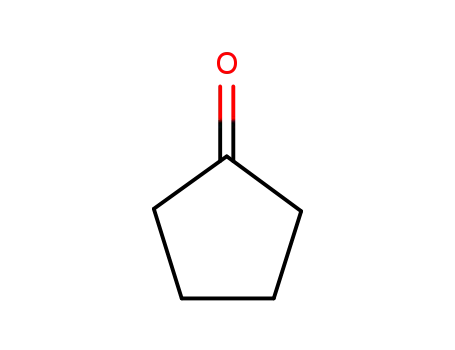
cyclopentanone
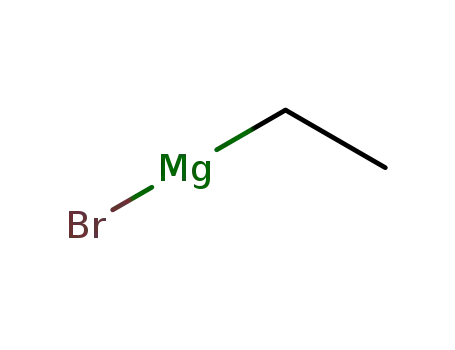
ethylmagnesium bromide
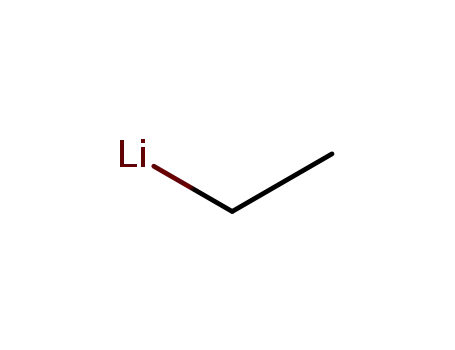
ethyllithium
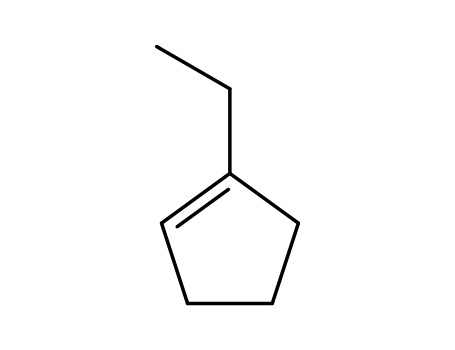
1-ethylcyclopentene
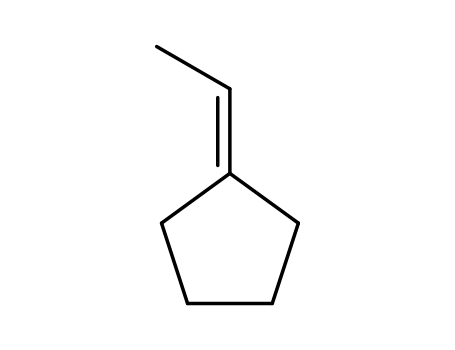
ethylidenecyclopentane
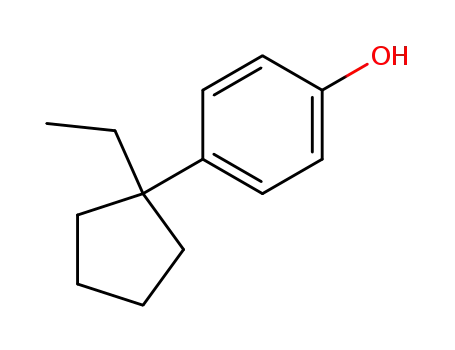
4-(1-ethyl-cyclopentyl)-phenol
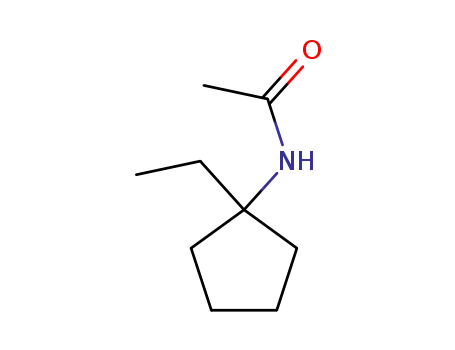
1-Ethyl-1-acetamido-cyclopentan
CAS:120-74-1
Molecular Formula:C<sub>8</sub>H<sub>10</sub>O<sub>2</sub>
Molecular Weight:138.16
CAS:1402393-56-9
Molecular Formula:C23H31BrO2
Molecular Weight:419.4
CAS:92343-46-9
Molecular Formula:C<sub>8</sub>H<sub>10</sub>O<sub>3</sub>
Molecular Weight:154.16