Your Location:Home >Products >OLED intermediates >Fluorenes >3096-56-8

Product Details
|
Chemical Properties |
yellow powder |
|
Uses |
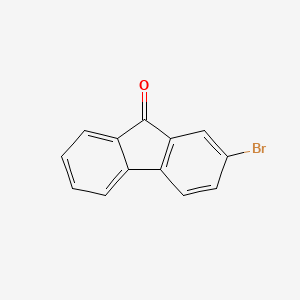
2-Bromo-9-fluorenone, a functional material as an organic intermediate. It is the best liquid crystal material and is used as an intermediate for OLED. |
|
Application |
2-Bromo-9-fluorenone was used:as end-capping agent for poly(9,9-dialkylfluorene-2,7-diyl) derivativesin synthesis of 2-bromo-9-hydroxy-9-(2-biphenyl)fluorenein preparation of spirobifluorene ligandsin synthesis of altitudinal molecular motors which contain functional groups in their rotor partin synthesis of FB |
|
Synthesis |
The synthesis of 2-Bromo-9-fluorenone is as follows:the 9-fluorenone (10mmol), the phase transfer catalyst (1.5mmol fourteen alkyl trimethyl ammonium chloride) and brominated aqueous ammonium solution (30 wt %, 25mmol ammonium bromide) mixing and heating system temperature to 75 °C, in 1h is divided into three time 11mmol potassium bromate, to three times the weight ratio of 1:3:1, to maintain the system temperature to continue reaction 3h, wherein the 9-fluorenone, ammonium bromide, the molar ratio of potassium bromate 1 : 2.5: 1.1 ; 9-fluorenone, and fourteen alkyl trimethyl ammonium chloride (phase-transfer catalyst) molar ratio of 1 : 0.15; 2) after the reaction, to room temperature, by adding 20% aqueous solution of sodium sulfite ( eliminates the bromine fluid ), is filtered, the filter cake washing water And dried to obtain yellow solid of 2-Bromo-9-fluorenone, the yield is 99.2%. |
|
Solubility in organics |
2-Bromo-9-fluorenone was 0.0526 (mole fraction) in 1,4-dioxane at 323.15 K and 0.0002 (mole fraction) in methanol at 278.15 K, respectively, and the solubility increased with the increase in temperature in all solvents. |
InChI:InChI=1/C13H7BrO/c14-8-5-6-10-9-3-1-2-4-11(9)13(15)12(10)7-8/h1-7H
This article deals with the saturated solubility and solvation behavior of 2-bromo-9-fluorenone in 10 industrial common solvents and three binary mixed solvents by means of experimental and mathematical correlations.
Knowledge of the local polarity of speci...
Twisted sisters: Transmission of chirali...
The oxidation of fluoren-9-ols by 4-nitr...
A photocatalyzed intramolecular cyclizat...
-
Herein, we report a novel solution proce...
Two new meso-tetrafluorenylporphyrin-cor...
-
We report the synthesis of altitudinal m...
-
The design of dendrimers featuring conju...
Electrochemical oxidation-induced direct...
The invention discloses a preparation me...
The invention provides an organic compou...
o-iodo-methyl-benzoic acid

9-fluorenone

2-bromofluoren-9-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: sodium carbonate; bis-triphenylphosphine-palladium(II) chloride / tetrahydrofuran; water / 12 h / 60 °C / Inert atmosphere
2: sodium hydroxide / water; methanol / 12 h / 20 °C
3: 1,2-bis-(diphenylphosphino)ethane; 2,2-dimethylpropanoic anhydride; chloro(1,5-cyclooctadiene)rhodium(I) dimer; potassium iodide / 40 h / 180 °C / Inert atmosphere; Sealed tube
With bis-triphenylphosphine-palladium(II) chloride; chloro(1,5-cyclooctadiene)rhodium(I) dimer; 2,2-dimethylpropanoic anhydride; sodium carbonate; 1,2-bis-(diphenylphosphino)ethane; potassium iodide; sodium hydroxide; In tetrahydrofuran; methanol; water;
|
methyl 4'-bromo-[1,1'-biphenyl]-2-carboxylate

9-fluorenone

2-bromofluoren-9-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sodium hydroxide / water; methanol / 12 h / 20 °C
2: 1,2-bis-(diphenylphosphino)ethane; 2,2-dimethylpropanoic anhydride; chloro(1,5-cyclooctadiene)rhodium(I) dimer; potassium iodide / 40 h / 180 °C / Inert atmosphere; Sealed tube
With chloro(1,5-cyclooctadiene)rhodium(I) dimer; 2,2-dimethylpropanoic anhydride; 1,2-bis-(diphenylphosphino)ethane; potassium iodide; sodium hydroxide; In methanol; water;
|
9-fluorenone
2-bromo-9H-fluorene
2-bromo-phenanthrene-9,10-dione
4-bromo-[1,1′-biphenyl]-2-carboxylic acid
2-amino-9-fluorenone
2-bromo-fluoren-9-one oxime
2-bromo-7-nitro-9H-fluoren-9-one
4'-bromo-[1,1'-biphenyl]-2-carboxylic acid